
许琛琦,研究员,1998年于华东师范大学获得生物化学专业学士,2004年于中科院生化细胞所获得生物化学与分子生物学博士学位,2004-2009年于哈佛大学Dana-Farber肿瘤研究所从事博士后研究,获得Arthritis Foundation资助,后晋升为Instructor。2009年底加入中科院生化与细胞所,入选国家基金杰出青年基金。阐明了抗原免疫应答调控的新机制并发展了肿瘤免疫治疗的新策略。工作已发表于Cell 2020, Nature 2018,Nature 2016,Nature 2013等,两项成果入选中国科学十大进展/中国生命科学十大进展,其发明专利已成功进入转化。荣获上海市青年科技杰出贡献奖(2020年),中国青年科技奖(2018年),中国生化与分子生物学会Promega生物化学奖(2018年)等荣誉。
免疫系统是机体执行免疫应答及免疫功能的重要系统,它能够区分“自身”和“非自身”,并通过清除“非自身物质”来保护机体。免疫系统可以分为固有免疫和适应性免疫。固有免疫系统在低等动物中就开始出现,可以识别病原体所携带的特殊模式以及体内的危险信号,从而迅速地做出免疫反应。适应性免疫系统是高等动物逐渐进化出的特殊功能系统,最大的特点是能够针对不同的抗原做出高度特异性和灵敏性的免疫反应。T淋巴细胞(简称T细胞)是适应性免疫系统的主要功能细胞,在清除病原体和肿瘤细胞过程中发挥着至关重要的作用。T细胞的活性异常与肿瘤、自身免疫病等多种重大疾病直接相关。基于T细胞功能调控的肿瘤免疫治疗已成为治疗肿瘤的主要武器之一,在临床上已取得了巨大的成功。但现有的基于信号转导调控的肿瘤免疫治疗手段只对部分病人有效,因此急需发展新的方法让更多的病人受益。本研究组交叉利用免疫学、生物化学、生物物理学前沿手段,着力于T细胞活性调控的分子机制研究,揭示了T细胞发挥免疫功能的分子基础,并且发展了新的肿瘤免疫治疗方法。
1. 抗原免疫应答的调控机制
抗原免疫应答过程涉及多种免疫受体的协同作用。本课题组对抗原受体TCR,共刺激受体CD28和共抑制受体PD-1的活化机制做了深入而系统的研究,我们创新性地提出了“膜脂调控”理论,指出酸性磷脂可以通过静电相互作用调控免疫受体的活化过程。
T细胞的活化主要依赖于抗原受体TCR(T-cell receptor)和共刺激受体CD28。我们的前期研究表明TCR的活性受酸性磷脂调控。带负电的酸性磷脂可以和带正电的TCR胞内区动态结合,从而将TCR的磷酸化位点屏蔽在膜内,保证TCR在静息态T细胞中处于功能关闭状态(Cell 2008)。我们随后发现TCR初始信号引发的钙离子内流能够反馈调控TCR。Ca2+可以直接结合酸性磷脂的磷酸根,中和其携带的负电荷,从而打破TCR与酸性磷脂之间的静电相互作用,促进TCR功能位点的解屏蔽及磷酸化,从而放大TCR的活化信号(Nature 2013)。这一新的分子机制突破了以往对Ca2+功能的传统认识。在这项工作基础上,我们进一步发现Ca2+的正反馈调控还适用于CD28。酸性磷脂同样可以屏蔽CD28的磷酸化位点,而Ca2+也能够通过中和酸性磷脂负电荷的机制来放大CD28信号(Nature Structural & Molecular Biology 2017)。我们由此提出TCR,Ca2+,CD28这三者之间组成了一个双环路的正反馈网络,可以将微弱的初始抗原刺激信号迅速放大,使T细胞获得完全的效应功能,从而为T细胞高灵敏性提供了信号基础(图1)。“膜脂调控”理论在我们的合作研究中同样被证明适用于多种免疫受体,比如钙离子在记忆型B细胞的迅速活化过程中也起到关键的作用(Nature Communications 2015,The Journal of Physical Chemistry Letters, 2017)。我们也撰写了多篇综述来阐述这个学术思想(Trends Biochem Sci 2014, Trends Immunol 2016, Nat Rev Immunol 2016)。
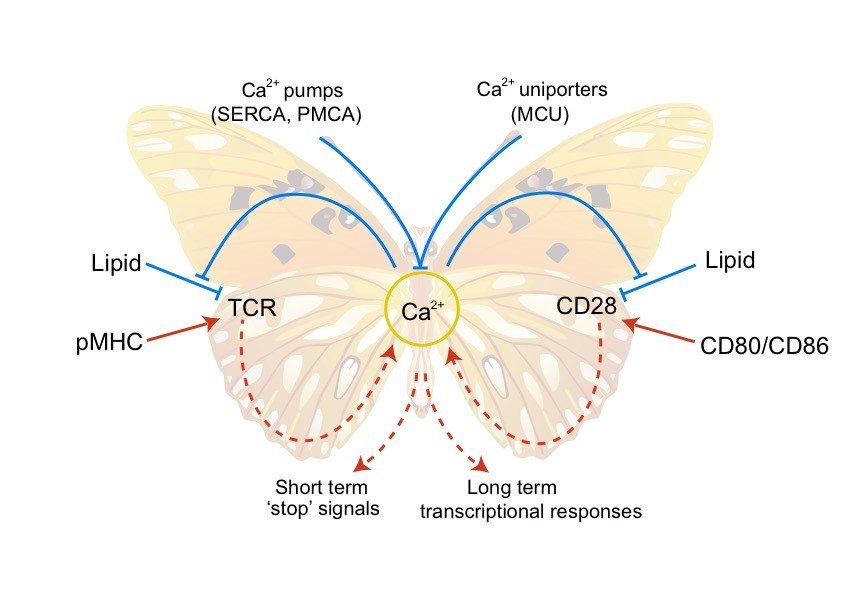
图1. TCR-Ca2+-CD28双环路正反馈模型。T细胞活化主要依赖TCR和CD28。这两个关键受体的磷酸化位点都被酸性磷脂通过静电效应屏蔽,而Ca2+可以通过中和酸性磷脂负电荷的形式释放受体磷酸化位点,促进受体活化。TCR和CD28的活化都能够诱导Ca2+内流。TCR-Ca2+-CD28这三者之间由此形成双环路的正反馈网络,可以将微弱的初始刺激信号迅速放大,为T细胞的高抗原敏感性提供信号基础。
我们还发现TCR的结构多态性和酪氨酸激酶Lck的底物选择性是T细胞高特异性的分子基础。通过单分子技术和液相核磁共振技术,我们发现TCR在不同的抗原刺激下会产生不一样的开放性构象,从而对Lck激酶有不同程度的招募;随后Lck对TCR上的多个酪氨酸位点进行选择性磷酸化,产生抗原特异性的磷酸化模式并引发不同的T细胞效应功能(图2,Cell Research 2017,PNAS 2017)。

图2. TCR结构多态性模型。TCR可以在不同抗原刺激下产生不同的开放性构象,从而引发特异性的下游信号通路,使得T细胞获得不同的效应功能。这个模型解释了T细胞获得抗原特异性的信号基础。
我们同样研究了T细胞的共抑制受体PD-1。T细胞在肿瘤微环境中会表现出功能耗竭的状态,并伴随着PD-1的异常高表达。目前在临床上应用的PD-1或PD-L1抗体就是通过阻滞PD-1的抑制性信号,进而增强T细胞的抗肿瘤活性。我们发现了PD-1的降解机制及其在抗肿瘤过程中的重要性 (Nature 2018)。活化T细胞表面的PD-1会经历内吞、泛素化和蛋白酶体的降解等一系列过程。我们找到了介导PD-1泛素化的E3连接酶FBXO38。在Fbxo38条件性敲除的小鼠体内肿瘤生长的会更快,肿瘤浸润T细胞的PD-1表达水平也更高。而PD-1抗体治疗有效抑制了FBXO38缺失小鼠体内肿瘤的生长,揭示了PD-1是FBXO38作用的直接靶点。FBXO38对PD-1表达量的调控为阻滞PD-1的抑制性信号提供了一个新的角度,也为针对PD-1的抗肿瘤药物的研发提供新的思路。
2. 肿瘤免疫治疗
近年来,本课题组利用自己在免疫受体和脂质调控方面的基础知识,提出了全新的肿瘤免疫治疗理论及策略。一方面我们认为通过调控T细胞的代谢状态可以让其获得更强的效应功能。我们发现胆固醇储存通路的关键调节酶ACAT1是一个很好的调控靶点,抑制ACAT1的活性可以大大提高CD8+ T细胞(又名杀伤性T细胞)的抗肿瘤功能。其机理是ACAT1被抑制后,杀伤性T细胞膜上的游离胆固醇水平提高,从而让TCR的聚集程度和信号转导能力提高并使得T细胞的杀伤性免疫突触形成更加有效。我们进一步利用ACAT1的小分子抑制剂Avasimibe在动物模型中治疗多种肿瘤,发现该抑制剂具有很好的抗肿瘤效应;并且Avasimibe与现有的肿瘤免疫治疗临床药物anti-PD-1联用后效果更佳。Avasimibe曾经作为心血管疾病的药物进行过三期临床试验,虽然这个小分子抑制剂对动脉粥样硬化没有明显的治疗效果,但是它具有很好的人体安全性,因此Avasimibe具有很好的潜力被开发成抗肿瘤药物(Nature 2016,中国生命科学十大进展)。
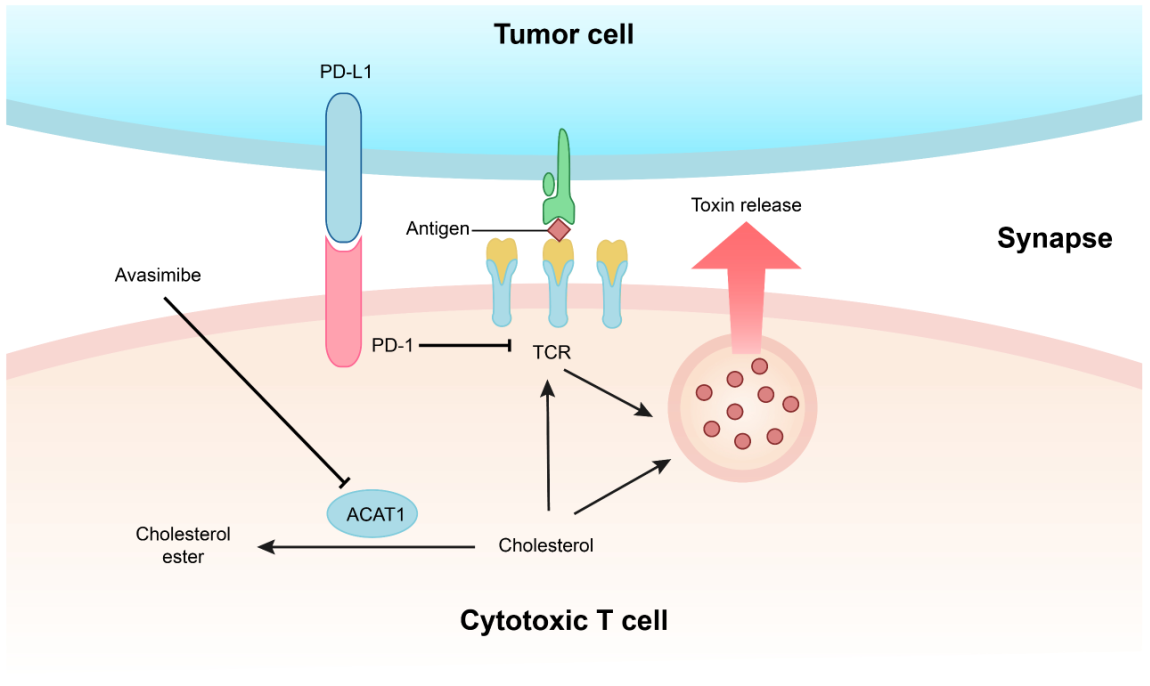
图3. 基于胆固醇代谢调控的肿瘤免疫治疗新方法。胆固醇酯化酶ACAT1可以将细胞内的游离胆固醇转化为胆固醇酯。抑制CD8+ T细胞的ACAT1活性可以使细胞质膜的游离胆固醇水平上升,从而使得TCR信号增强并让杀伤性免疫突触更成熟。T细胞肿瘤抗原免疫应答由此变得更加高效。
另一方面我们基于CD3e 蛋白的多重信号功能发展了一种新的CAR-T细胞治疗技术,可以提高细胞的抗肿瘤功能并降低细胞因子风暴的风险。通过建立绝对定量质谱系统,我们对CD3分子内的所有酪氨酸位点定量并发现CD3e ITAM呈现出独特的单磷酸化的模式。进一步的研究发现,CD3e ITAM可以特异性的招募抑制性激酶Csk来抑制Lck介导的磷酸化过程,表明天然的抗原受体TCR同时具有活化元件(CD3z)和调控元件(CD3e),具有信号自我调控的能力。目前临床上使用的CAR-T细胞,其人工合成的抗原受体CAR却只利用了CD3z。当我们将CD3e加入至临床上使用的CAR中后,CAR-T细胞的抗肿瘤能力得到了增强。从机制的角度来看,CD3e中的ITAM基序可以招募Csk来下调CAR-T细胞的细胞因子的分泌水平,BRS基序可以招募p85进而增强CAR-T细胞的生长持续性。因此在CAR中加入了CD3e可以引入更丰富的信号调控机制,从而提升CAR-T的整体表现,为未来的CAR-T细胞治疗提供新的发展方向(Cell 2020,中国生命科学十大进展)。
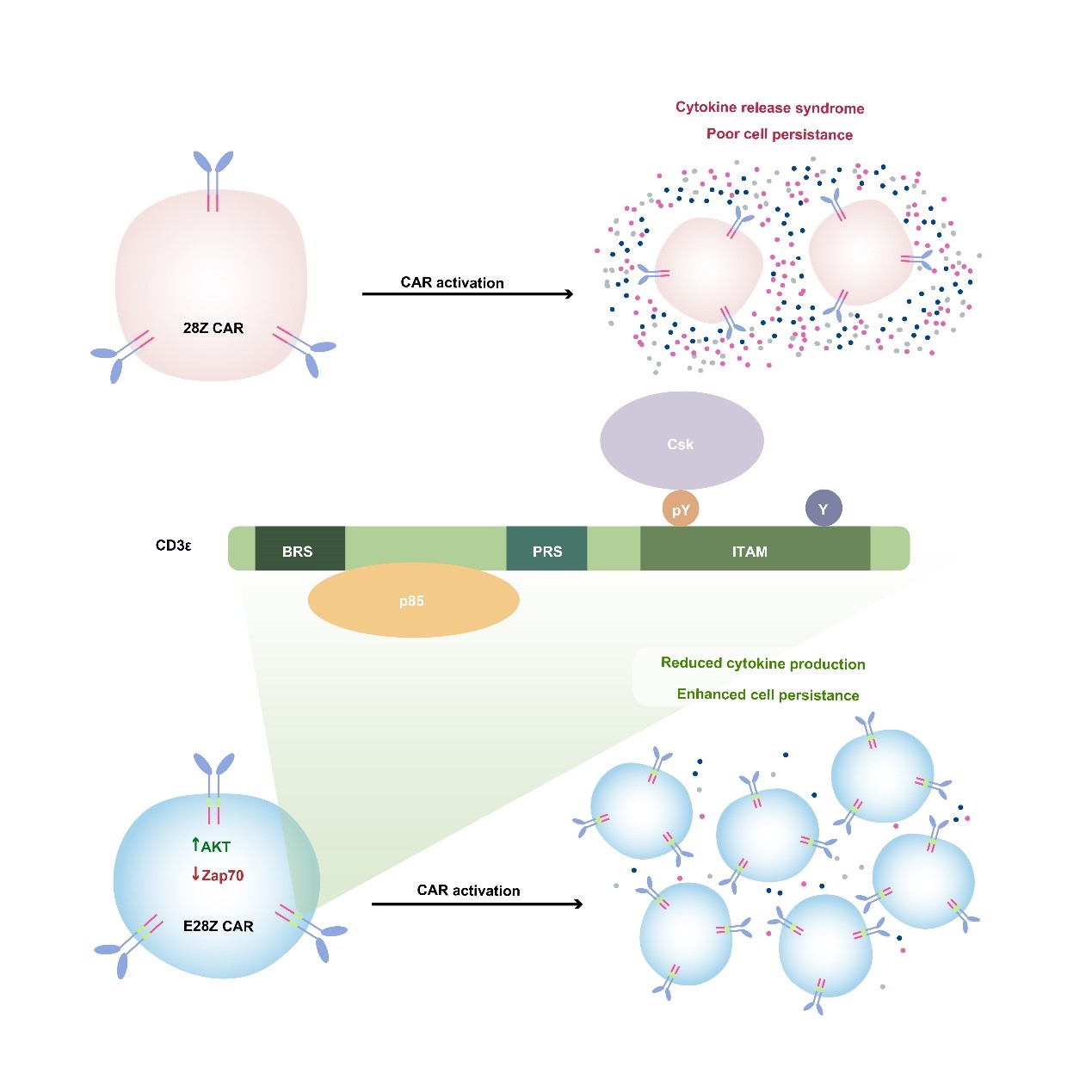
图4. 基于CD3e 蛋白多重信号功能而设计的新型CAR-T细胞疗法。TCR的CD3e 链具有特殊的信号转导功能,可以同时招募抑制性分子Csk和活化性分子PI3K。将CD3e 胞内区加入临床使用的CAR序列中,可使得CAR-T细胞生长持续性更好,抗肿瘤功能更强,并且细胞因子释放综合症的风险降低。
综上所述,本研究组在T细胞的基础研究中发现了T细胞活化的新分子机制,同时在免疫受体信号和代谢调控两个角度发展了新的肿瘤免疫治疗方法。今后我们将主要研究在不同生理和病理环境下的T细胞脂代谢特征,并且寻找新的“代谢检查点”用于代谢调控,同时进行CAR-T细胞的功能机制研究,为未来CAR-T细胞的设计与临床应用提供新的思路与发展方向。

合影